COVID-19 drug study underway. Florida communities invited to join.
Thriving Mind South Florida invites Florida communities to learn more and consider participation in a research project offered by Washington University School of Medicine in St. Louis. Researchers there are testing a medicine for patients with mild symptoms in the early course of COVID-19 treatment.
As recently reported on CNN, the purpose of this study, the STOP COVID Trial, is to slow the progression of COVID-19 and prevent hospitalization.
Participants will be provided with free supplies, with free phone and internet follow-up, and they will be compensated with $50 for their time. All participation is from home, with no doctor’s visit required.
About the study
The study is contactless, meaning volunteers can participate without leaving home. Please see below and the attached flyer and IRB approval. Participants get free supplies (such as pulse oximeter), with free phone and internet follow-up, and are compensated with $50.
About the research team
The Principal Investigator, Eric Lenze, MD, a faculty member at Washington University in St. Louis and the lead coordinator, Julie Schweiger, are highly experienced colleagues who Thriving Mind CEO John W. Newcomer, M.D., has worked with for over 25 years.
To enroll
Go to the study website at http://stopcovidtrial.wustl.edu, or email stopcovidtrial@wustl.edu with the potential participant’s name and phone number, or call 314-747-1137. You will get a response within 24 hours, if not immediately.
Key points
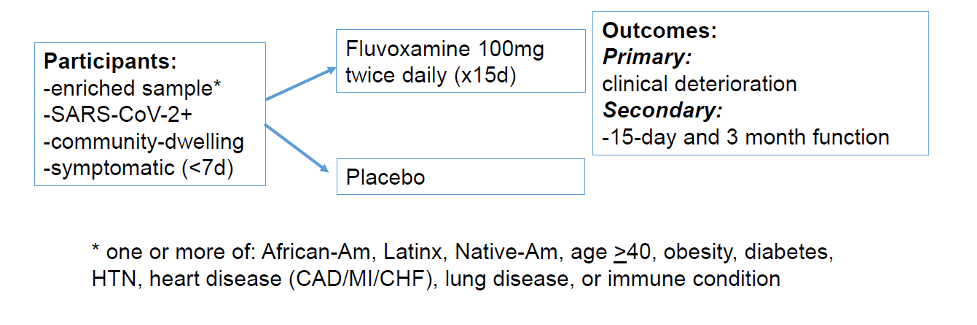
- This same research team previously showed that fluvoxamine prevented clinical deterioration (dyspnea and hypoxia). That peer-reviewed study was published in the Journal of the American Medical Association.
- This new study aims to confirm those findings in a larger, national group of people in the US.
- Researchers want individuals to enroll in a new study that will test fluvoxamine’s benefits for COVID.
- This is an at-home clinical trial. The study team sends you the medication and everything you need to participate.
- The study enrolls from anywhere in the US.
- Every participant received free supplies (such as pulse oximeter) and gets paid $50.
Regulatory details
This is a Washington University Institutional Review Board (IRB)-approved protocol that does not involve “engagement” of local staff (no local IRB approval required). Information here can be shared with anyone in the community and patients with positive results can be referred to the number or email provided in the IRB approved flyer to get more information.
If people call or contact them, the St Louis-based team will do the screening and any appropriate enrollment, get consent and ship the materials as well as do all needed weekly follow up.